In a rapidly evolving landscape of technological innovation, recent developments signal both the promise and the perils of the future.From the dawn of autonomous mobility to breakthroughs in artificial intelligence-driven medicine, these advancements are reshaping industries and redefining possibilities. Yet, as Tesla’s Robotaxi faces its first collision and Google’s AI-powered pharmaceutical prepares for its first human trials, questions about safety, regulation, and trust come sharply into focus. Simultaneously occurring, Hong Kong’s ambition to issue a stablecoin license within the year highlights the ongoing push toward financial digitalization. Together, these stories offer a compelling glimpse into a world on the cusp of transformation—where technological progress is poised to revolutionize daily life, even as it prompts careful consideration of the challenges ahead.
Emerging Challenges in Autonomous Vehicle Safety and Regulatory Responses
As autonomous vehicles become more commonplace, safety concerns and regulatory hurdles are increasingly at the forefront. The recent incident involving Tesla’s Robotaxi, which encountered its first collision, underscores the challenges in ensuring reliable perception and decision-making systems. Regulators worldwide are grappling with questions such as:
- How should autonomous vehicle behavior be standardized?
- What are the minimum safety benchmarks before deployment?
- How to integrate real-time data to enhance safety protocols?
Meanwhile, technological advances like Google’s AI-powered drug discovery are pushing the boundaries of innovation, but also raising ethical and safety considerations for human trials. Regulatory bodies are responding by establishing frameworks that balance innovation with public health. The following table highlights emerging areas of concern and how regulators are addressing them:
| Challenge | Regulatory Response | Progress |
|---|---|---|
| Vehicle cybersecurity | Enhanced standards & auditing | Ongoing |
| AI Trial Safety | Ethical review boards & phased testing | Implementing pilot programs |
| Digital Currency licensing | Clear licensing frameworks & compliance checks | initial licenses issued |
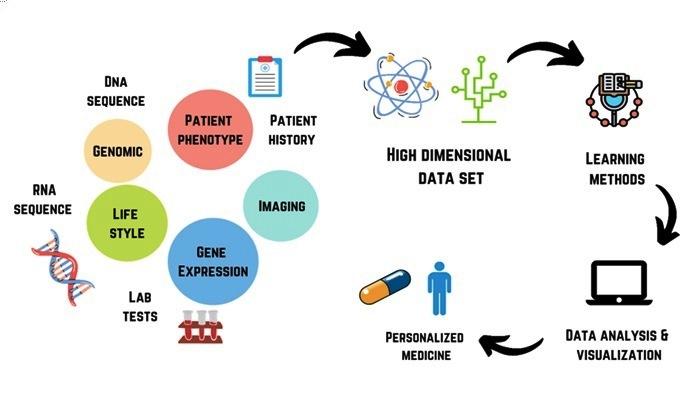
Pioneering Advances in AI-Driven medicine and the Path Toward Clinical Validation
Recent innovations in AI-driven medicine are rapidly transforming the landscape of healthcare. Cutting-edge algorithms now assist in diagnosing complex conditions with unprecedented accuracy, and machine learning models are optimizing treatment plans personalized to individual genetic profiles. However, the journey from breakthrough to bedside remains intricate, requiring rigorous clinical validation to ensure safety and efficacy. As research teams pioneer new methodologies, collaboration between technologists and clinicians becomes vital to bridge the gap between experimental success and real-world application.
key milestones in this domain include:
- First triumphant clinical trials evaluating AI-predicted drug interactions.
- Implementation of automated diagnostic tools in hospitals nationwide.
- Growth of regulatory frameworks supporting AI innovation while safeguarding patient health.
| Stage | Focus area | Current Status |
|---|---|---|
| Discovery | Genomic Data Analysis | Rapid advancements |
| Preclinical | Drug Simulation | Increasing accuracy |
| Clinical Validation | Patient Trials | Initial phases underway |

Hong Kong’s Strategic Approach to Stablecoin Licensing and Financial Innovation
Hong Kong continues to position itself as a nexus for innovative financial technology, leveraging a strategic approach that balances regulation with growth. The region’s targeted licensing framework for stablecoins exemplifies this vision, aiming to create a robust yet flexible habitat for startups and established players alike.By prioritizing transparency and compliance, Hong kong strives to attract global crypto firms, fostering a vibrant ecosystem where financial innovation can flourish without compromising stability or investor protection.
To achieve this, authorities have outlined clear steps:
- Streamlined licensing procedures to reduce barriers for new entrants
- Collaborative regulatory sandboxing to test innovative products in real-world scenarios
- Ongoing dialog with industry leaders to refine policies and adapt to emerging trends
| Focus Area | Key Initiative |
|---|---|
| Legal Clarity | Clear licensing guidelines for stablecoins |
| Innovation Support | Financial tech sandbox programs |
| Global Engagement | Partnerships with international regulators |
Closing Remarks
As we stand at the crossroads of innovation, these milestones reveal a landscape that’s both promising and complex. From the first collision involving Tesla’s Robotaxi to Google’s breakthrough in AI-driven medicine, and Hong kong’s stride toward establishing a stablecoin license—each reflects the rapid evolution shaping our future. While these developments hold great potential,they also serve as reminders of the challenges ahead,urging us to navigate this technological frontier with cautious optimism and thoughtful stewardship. As the journey continues, staying informed and engaged will be key to shaping a smarter, safer world.